Perkembangan ilmu pengetahuan dan teknologi telah mewarnai pengembangan obat-obatan terutama dalam bidang formulasi, teknologi produksi, dan pengawasan mutu obat yang telah melibatkan aplikasi peralatan dan metode yang semakin canggih dan kompleks. Hal ini juga telah diikuti dengan perkembangan regulasi dalam bidang kefarmasian, khususnya regulasi dalam bidang industri. Proses globalisasi dalam berbagai dimensi kehidupan, juga berdampak terhadap terjadinya globalisasi dalam bidang farmasi melalui harmonisasi pada tingkat regional dan global.
Menghadapi tantangan ini, dibutuhkan tenaga-tenaga farmasis profesional yang memiliki bekal kemampuan untuk mengatasi berbagai masalah yang dihadapi industri farmasi. Kemampuan yang dituntut dari seorang farmasis oleh industri farmasi dewasa ini hanya dapat dipenuhi melalui pemberian dasar keilmuan kefarmasian yang kokoh, yang difokuskan pada aspek-aspek yang berhubungan dengan aspek produksi dan pengawasan mutu obat.
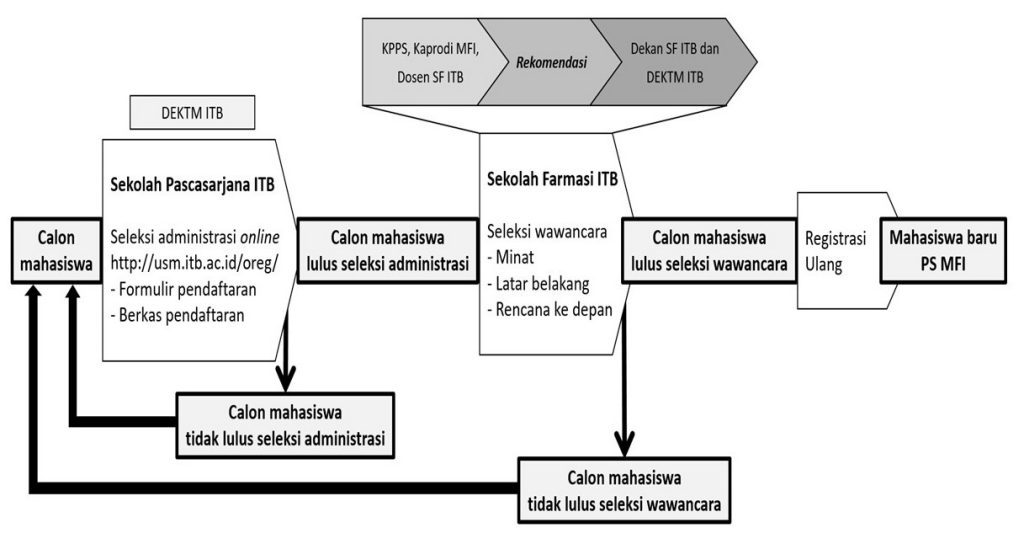
Sekolah Farmasi ITB telah membuka Program Studi Magister Farmasi Industri sebagai upaya mempersiapkan sumberdaya manusia yang memiliki kompetensi lebih bagi industri farmasi. Program Studi Magister Farmasi Industri didukung oleh kepakaran dan jaringan yang kuat serta kemitraan ITB dengan industri farmasi dan badan otoritas regulasi, baik dalam maupun luar negeri.
Visi
Menjadi Program Pendidikan Magister Farmasi Industri yang unggul dan bermartabat secara nasional dan internasional.
Misi
- Menyelenggarakan Pendidikan Farmasi Industri sesuai dengan perkembangan iptek terkini terutama iptek dalam bidang farmasi industri.
- Menyelenggarakan penelitian terapan yang inovatif, kompetitif dan berkesinambungan dalam bidang farmasi industri.
- Menerapkan hasil-hasil penelitian dan pengetahuan terkini dalam kegiatan pengabdian kepada masyarakat.
- Menjalin kerja sama intensif dan mendayagunakan industri farmasi dan kosmetik nasional maupun internasional serta sumber daya terkait untuk pengembangan industri farmasi dan kosmetik di Indonesia.
Tujuan Program Studi
- Menghasilkan lulusan yang memiliki integritas yang tinggi, kemampuan profesional dalam mengelola berbagai kegiatan rutin di industri farmasi, yang mencakup tugas-tugas: perancangan pabrik farmasi, penentuan formula dan teknik pembuatan sediaan farmasi, penentuan spesifikasi dan metode standardisasi, pembuatan/produksi sediaan farmasi dan pengendaliannya, pengawasan dan penjaminan mutu, pengemasan, penetapan kondisi penyimpanan dan usia simpan produk, pengelolaan bahan awal dan obat jadi, pendaftaran obat jadi, partisipasi dalam menghasilkan dan diseminasi inovasi dan teknologi baru.
- Menghasilkan lulusan yang mampu menyelesaikan masalah-masalah dalam produksi dan pengujian mutu sediaan farmasi di lapangan secara tepat dan cepat (terarah) berdasarkan pertimbangan ilmiah yang mendalam dan dengan memperhatikan berbagai aspek secara komprehensif, termasuk di dalamnya adalah pengembangan formulasi, standardisasi baik untuk obat lama maupun obat baru, dan perkembangan regulasi terkini.
- Menghasilkan lulusan yang berkontribusi terhadap pengembangan IPTEK farmasi di industri skala nasional maupun internasional berupa penelitian dan pengembangan produk-produk baru (obat, kosmetik, produk biomedik, nutrasetikal, suplemen makanan-minuman, jamu, dan obat tradisional) dan standardisasi sediaan farmasi.